Results That Matter to Patients
Comprehensive data to guide your treatment choices
APHENITY Study: From Identification to Confirmation
Two-part design: Identify responders, then confirm efficacy1,2
SEPHIENCE was evaluated in a two-part, global, double-blind, randomized, placebo-controlled study in patients with PKU. The primary efficacy was assessed by the mean change in blood Phe levels from baseline to Week 6 in the SEPHIENCE-treated group compared to the placebo group.
Consistent and Significant Phe Reduction1,2
APHENITY: Results observed across all ages, from pediatrics to adults2
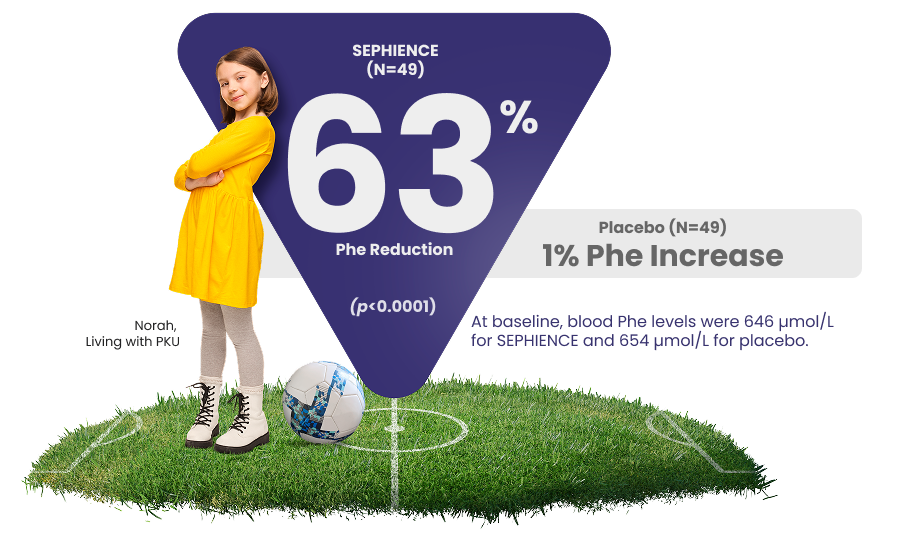
At baseline, blood Phe levels were 646 µmol/L for SEPHIENCE and 654 µmol/L for placebo.
Additional Efficacy Analyses
The following analyses were not prespecified in the Phase 3 trial and are not included in the approved Prescribing Information for SEPHIENCE. Always refer to the full Prescribing Information for SEPHIENCE use. See additional study details below for more information.
Considerable Responsiveness Across Subgroups
APHENITY subgroup analysis was consistent with the results of the primary efficacy2
Mean % Change in Blood Phe from Baseline to Week 6 in Part 22,3
Classical PKU
BH4 Non-Responsive
SEPHIENCE (N=6)
-69%
SEPHIENCE (N=13)
-54%
Placebo (N=9)
+3%
Placebo (N=10)
-12%
(p<0.0001)
(p=0.001)

At baseline, blood Phe levels were 761 µmol/L for SEPHIENCE and 772 µmol/L for placebo in patients with classical PKU, and 650 µmol/L for SEPHIENCE and 662 µmol/L for placebo in those who were BH4 non-responsive.2
The mean absolute change in blood Phe from baseline to Week 6 was greater with SEPHIENCE than with placebo in both Classical PKU (-523 μmol/L vs -42 μmol/L) and BH4 non-responsive patients (-354 μmol/L vs -78 μmol/L).2
Additional study details2
Muntau et al. 2024 Study Summary
Efficacy and safety of oral sepiapterin in patients with phenylketonuria (APHENITY)
A phase 3, double-blind, randomized, placebo-controlled study in participants with PKU aged
2 years and older across 34 sites in 13 countries.
The primary efficacy endpoint was mean change in blood Phe concentration from baseline to Week 6. Sepiapterin significantly reduced blood Phe concentration compared to placebo (least squares mean difference: –395.9 μmol/L; p<0.0001). Secondary analyses showed that 93% of participants with a baseline blood Phe level of ≥600 µmol/L and 84% with a baseline level of ≥360 µmol/L achieved target Phe levels with sepiapterin, compared to 30% and 9% with placebo, respectively (both p<0.0001). Additionally, 22% of sepiapterin-treated participants achieved normal Phe levels (35‑120 μmol/L) compared to none with placebo.
Sepiapterin was generally well tolerated. The most common treatment-emergent adverse events were mild gastrointestinal symptoms, with no deaths, serious adverse reactions, or severe adverse events reported.
Study Limitations
Only participants who were sepiapterin-responsive (≥15% Phe reduction) were randomized, and the study duration was limited to 6 weeks.
Financial Disclosures of Study Sponsors
Funded by PTC Therapeutics.
Muntau AC, Longo N, Ezgu F, et al. Effects of oral sepiapterin on blood Phe concentration in a broad range of patients with phenylketonuria (APHENITY): results of an international, phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2024;404(10460):1333-1345.
Achieving Guideline-Target Blood Phe Levels
APHENITY: Percentage of patients who reached US guideline target levels (all ages)

US guidelines for all ages2
84%
of patients treated with SEPHIENCE reached ≤360 μmol/L (n=37/44)*
22%
of patients treated with SEPHIENCE had blood Phe levels within the normal range (n=11/49) vs 0% (n=0/49) of patients with placebo
Additional study details2
Muntau et al. 2024 Study Summary
Efficacy and safety of oral sepiapterin in patients with phenylketonuria (APHENITY)
A phase 3, double-blind, randomized, placebo-controlled study in participants with PKU aged 2 years and older across 34 sites in 13 countries.
The primary efficacy endpoint was mean change in blood Phe concentration from baseline to Week 6. Sepiapterin significantly reduced blood Phe concentration compared to placebo (least squares mean difference: –395.9 μmol/L; p<0.0001). Secondary analyses showed that 93% of participants with a baseline blood Phe level of ≥600 µmol/L and 84% with a baseline level of ≥360 µmol/L achieved target Phe levels with sepiapterin, compared to 30% and 9% with placebo, respectively (both p<0.0001). Additionally, 22% of sepiapterin-treated participants achieved normal Phe levels (35‑120 μmol/L) compared to none with placebo.
Sepiapterin was generally well tolerated. The most common treatment-emergent adverse events were mild gastrointestinal symptoms, with no deaths, serious adverse reactions, or severe adverse events reported.
Study Limitations
Only participants who were sepiapterin-responsive (≥15% Phe reduction) were randomized, and the study duration was limited to 6 weeks.
Financial Disclosures of Study Sponsors
Funded by PTC Therapeutics.
Muntau AC, Longo N, Ezgu F, et al. Effects of oral sepiapterin on blood Phe concentration in a broad range of patients with phenylketonuria (APHENITY): results of an international, phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2024;404(10460):1333-1345.
APHENITY Extension: Evaluating Long-Term Phe Tolerance and Safety
Interim results: Increased dietary Phe intake4
N=169 enrolled as of September 2, 2024; 102 with blood Phe <360 µmol/L entered a 26-week dietary assessment phase.
Study Limitations: APHENITY Extension Study is an ongoing, open-label extension study with self-reported dietary protein intake. Missing diary records, wide age range, and a heterogeneous population may bias dietary Phe intake results. While the APHENITY Extension Study cannot determine whether evidence supports dietary Phe liberalization, it demonstrates the durability of SEPHIENCE’s effect in maintaining blood Phe <360 µmol/L, its long-term safety, and the potential for diet liberalization in some adults and children with PKU. Data are for informational purposes only and should be interpreted with caution. Individual results may vary.
In the dietary Phe assessment group:

of patients increased
their Phe intake4
Nearly all patients (n=99/102) were able to increase their dietary Phe intake at any time during the 26-week assessment. During the trial, 73% (n=74/102) of patients doubled and 34% (n=35/102) tripled their baseline Phe intake.4

met their
age-adjusted RDA4
Two-thirds of patients (n=57/81) reached their RDA for natural protein while on SEPHIENCE.4

protein/day by Week 264
A 110-lb (50 kg) patient could consume approximately 60 grams of dietary protein per day after 26 weeks of treatment.4*
Results are from a prespecified, interim analysis and should be interpreted accordingly.
*Based on an estimated conversion (1 g protein = 50 mg Phe).4
Additional study details4
van Spronsen et al. 2025 Study Summary
Long-term safety and dietary phenylalanine tolerance with oral sepiapterin in patients with phenylketonuria (APHENITY Extension Study)
A phase 3, ongoing, open-label extension study is evaluating the long-term safety of sepiapterin and its impact on dietary Phe tolerance in children and adults with PKU. As of the September 2, 2024 data cutoff, 169 participants (aged ~2 months to 55 years; median age 14 years) enrolled and received sepiapterin daily for ≥1 year. Participants were stratified based on blood Phe levels at the end of the first 2 weeks. Group 1 (n=102) had a mean blood Phe <360 μmol/L and entered a 26-week diet assessment phase where dietary Phe intake was gradually increased under close monitoring. Group 2 (n=49) had a mean blood Phe ≥360 μmol/L and continued sepiapterin without the dietary Phe tolerance assessment.
The primary efficacy endpoint is the mean change in dietary Phe intake from baseline to Week 26 in participants with blood Phe <360 μmol/L after the first 2 weeks (n=102). Primary safety endpoints include treatment-emergent adverse events, clinical laboratory tests, vital signs, and physical examinations. Sepiapterin increased dietary Phe tolerance, with mean dietary Phe intake increasing from 27.6 to 62.5 mg/kg/day at Week 26 (least squares mean difference: 36.4 mg/kg/day from baseline; p<0.0001). Nearly all participants (97%) increased dietary Phe intake during the 26-week assessment period, with 73% doubling and 34% tripling intake from baseline.
The long-term safety profile of sepiapterin was consistent with that observed in the APHENITY trial. Treatment-related adverse events occurred in 29% of participants. The most commonly reported events were headache (8.3%), diarrhea (7.7%), discolored feces (4.1%), vomiting (3.0%), and fatigue (2.4%). Three participants (1.8%) discontinued due to adverse events, and no treatment-related serious adverse events or deaths occurred.
Financial Disclosures of Study Sponsors
Funded by PTC Therapeutics.
van Spronsen F, Peters H, Margvelashvili L, et al. Effect of long-term sepiapterin treatment on dietary phenylalanine tolerance in patients with phenylketonuria: interim results from the Phase 3 APHENITY Extension Study. Genet Med. Publication accepted Nov, 2025.
Consistent Safety Profile Across Age Groups
APHENITY Safety: Most adverse reactions were mild or moderate1,2
Warnings and Precautions:
- Increased Bleeding: SEPHIENCE may increase the risk of bleeding. Consider treatment interruption in patients with active bleeding.
- Hypophenylalaninemia: Some pediatric PKU patients experienced hypophenylalaninemia; monitor patients’ blood Phe levels during treatment
- Interaction with Levodopa: Seizures, over-stimulation, or irritability may occur; monitor patients for a change in neurologic status
Adverse Reactions That Occurred in ≥2% of SEPHIENCE-Treated Patients and Greater Than in Placebo (APHENITY, Part 2)1
Slide table to view more
Adverse Reaction
(Preferred Term)
Diarrhea
Headache
Abdominal pain*
Hypophenylalaninemia
Feces discolored
Oropharyngeal pain
SEPHIENCE (%)
N=56
4 (7)
4 (7)
3 (5)
2 (4)
2 (4)
2 (4)
Placebo (%)
N=54
1 (2)
1 (2)
1 (2)
0
0
1 (2)
*Includes abdominal pain, abdominal pain upper, and abdominal discomfort.
Proven safety profile with no serious adverse reactions reported1,2
No treatment-related serious adverse events were reported in SEPHIENCE-treated PKU patients in clinical trials.1,2
Adverse reactions were similar across both adult and pediatric populations, except for hypophenylalaninemia, which was observed in 5% (n=2/37) of pediatric patients but not in any adult patients in APHENITY Part 2.1
The safety profile of SEPHIENCE up to 2.6 years in 169 participants was consistent with that observed in the APHENITY trial4
- The most common treatment-emergent adverse events (≥2%), were headache (8.3%), diarrhea (7.7%), discolored feces (4.1%), vomiting (3.0%), and fatigue (2.4%)4
- The safety profile remained consistent over time, with no safety concerns emerging with long-term use4
Stay informed
Sign up to receive updates, clinical insights, and resources straight to your inbox.
BH4: Tetrahydrobiopterin; CI: Confidence interval; OR: Odds ratio; Phe: Phenylalanine; PKU: Phenylketonuria; RDA: Recommended daily allowance.
References: 1. SEPHIENCE. Package Insert. PTC Therapeutics, Inc; 2025. 2. Muntau AC, Longo N, Ezgu F, et al. Effects of oral sepiapterin on blood Phe concentration in a broad range of patients with phenylketonuria (APHENITY): results of an international, phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2024;404(10460):1333–1345. doi:10.1016/S0140‐6736(24)01556‐3 3. Data on file. PTC Therapeutics, Inc; 2025. 4. van Spronsen F, Peters H, Margvelashvili L, et al. Effect of long-term sepiapterin treatment on dietary phenylalanine tolerance in patients with phenylketonuria: interim results from the Phase 3 APHENITY Extension Study. Genet Med. Publication accepted Aug, 2025.
You are now leaving the SEPHIENCE website and will enter a website operated by an independent third party. The links to third-party websites contained on this website are provided solely for your convenience. PTC Therapeutics does not control the opinions, claims, or comments on any third-party website linked to this website; the policies and practices of those third parties will govern your activities at those websites.
